
Biosafety Policy – May 29, 2025
Page 1 of 3
BIOSAFETY
POLICY
Policy Type: Management
Initially
Approved:
Apr 4, 2011
Policy
Sponsor:
Provost & Vice-President,
Academic
Last
Revised:
May 29, 2025
Primary
Contact:
AVP, Research, Scholarship
and Community
Engagement
Review
Scheduled: May 29, 2030
Approver:
Board of Governors
A.
OVERVIEW
Mount Royal University is committed to high standards for health and safety in teaching and research. To
ensure that this commitment is maintained, the University will establish an Institutional Biosafety
Committee (IBC) to facilitate teaching and research compliant with the Human Pathogens & Toxins Act and
Regulation (HPTA/R), and Public Health Agency of Canada (PHAC) biosafety standards and guidelines.
B.
PURPOSE
This Policy establishes the requirements for research and teaching activities that involve Pathogens, which
includes:
a)
Possessing, handling or use of a human pathogen or toxin;
b)
Producing a human pathogen or toxin;
c)
Storing a human pathogen or toxin;
d)
Permitting any person access to a human pathogen or toxin;
e)
Transferring a human pathogen or toxin;
f)
Importing or exporting a human pathogen or toxin;
g)
Relaxing or otherwise abandoning a human pathogen or toxin; or
h)
Disposing of a human pathogen or toxin.
C.
SCOPE
This Policy applies to all Members of the University Community that work with Pathogens defined within
the Human Pathogens & Toxins Act and Regulation.
D.
POLICY STATEMENT
1.
GENERAL
1.1
The University is committed to providing, promoting and maintaining a safe and healthy
environment for research and learning with Pathogens in accordance with the Canadian
Biosafety Standard and Handbook.
1.2
No teaching or research project that involves Pathogens may commence without prior IBC
or Biosafety Officer approval of a written protocol.
1.3
All teaching and research involving Pathogens are subject to ongoing monitoring by the IBC
or Biosafety Officer.
2.
RESPONSIBILITY

Biosafety Policy – May 29, 2025
Page 2 of 3
2.1
The Provost and Vice-President, Academic delegates authority regarding the
implementation of this Policy to the Associate Vice-President, Research, Scholarship and
Community Engagement.
2.2
The Associate Vice-President, Research, Scholarship and Community Engagement is
responsible for oversight of the University’s Biosafety Program including acting as the
License Holder for the University’s PHAC Pathogens and Toxins License.
2.3
The University will establish and maintain an IBC that will operate under Terms of Reference
to promote and monitor compliance with the provisions of the HPTA/R, Pathogens and
Toxins License conditions, and with the applicable health and safety, and biosecurity
standards.
2.4
The Biosafety Officer is assigned the responsibility of maintaining, supporting and
developing the Biosafety Program.
2.5
The IBC Chair will ensure maintenance of a confidential repository of all applications,
decisions, minutes and other materials pertaining to the business of the IBC.
3.
NON-COMPLIANCE
3.1
Pathogen use that has not been reviewed and approved by the IBC or Biosafety Officer
contravenes this Policy and constitutes non-compliance.
3.2
Non-compliance of this Policy will be managed as per section 4 of the Procedure for
Biosafety Management.
E.
DEFINITIONS
(1)
Policy:
means the Biosafety Policy.
(2)
University:
means Mount Royal University.
(3)
IBC
means the Institutional Biosafety Committee.
(4)
EH&S:
means the Environmental Health & Safety department.
(5)
Pathogen:
means a human pathogen which is a micro-organism, nucleic acid or
protein as defined in Section7 of the Human Pathogens and Toxins
Act.
(6)
Member of the
University Community:
means any individual who teaches, studies, conducts research; all
Employees, contractors, volunteers, and visitors to the University;
and any other individual acting on behalf of the University.
(7)
License Holder
means the person designated according to the definition within the
Human Pathogens and Toxins Act.
(8)
Biosafety Program
means any document related to pathogen and biohazardous
material use, handling or disposal at the University.
(9)
Biosafety Officer:
means the person within EH&S designated according to the
definition within the Human Pathogens and Toxins Act.
F.
RELATED POLICIES
● Procedure for Biosafety Management
● Environmental Health and Safety Policy
● Responsible Conduct in Research Policy
G.
RELATED LEGISLATION
● Environmental Protection Act, Canada
Biosafety Policy – May 29, 2025
Page 3 of 3
● Occupational Health and Safety Act & Code, Alberta
● Human Pathogens and Toxins Act & Regulation, Canada
● Transportation of Dangerous Goods Act, Canada
H.
RELATED DOCUMENTS
● Biosafety Manual, MRU
● Canadian Biosafety Standard and Handbook (current version; PHAC)
● Chemical and Biological Waste Disposal Manual, MRU
● Environmental Health & Safety Management System Manual, MRU
● Health Canada Laboratory Biosafety Guideline, 3
rd
Edition, 2004
● Institutional Biosafety Committee Terms of Reference
● Natural Sciences and Engineering Research Council of Canada (NSERC) Guidelines
I.
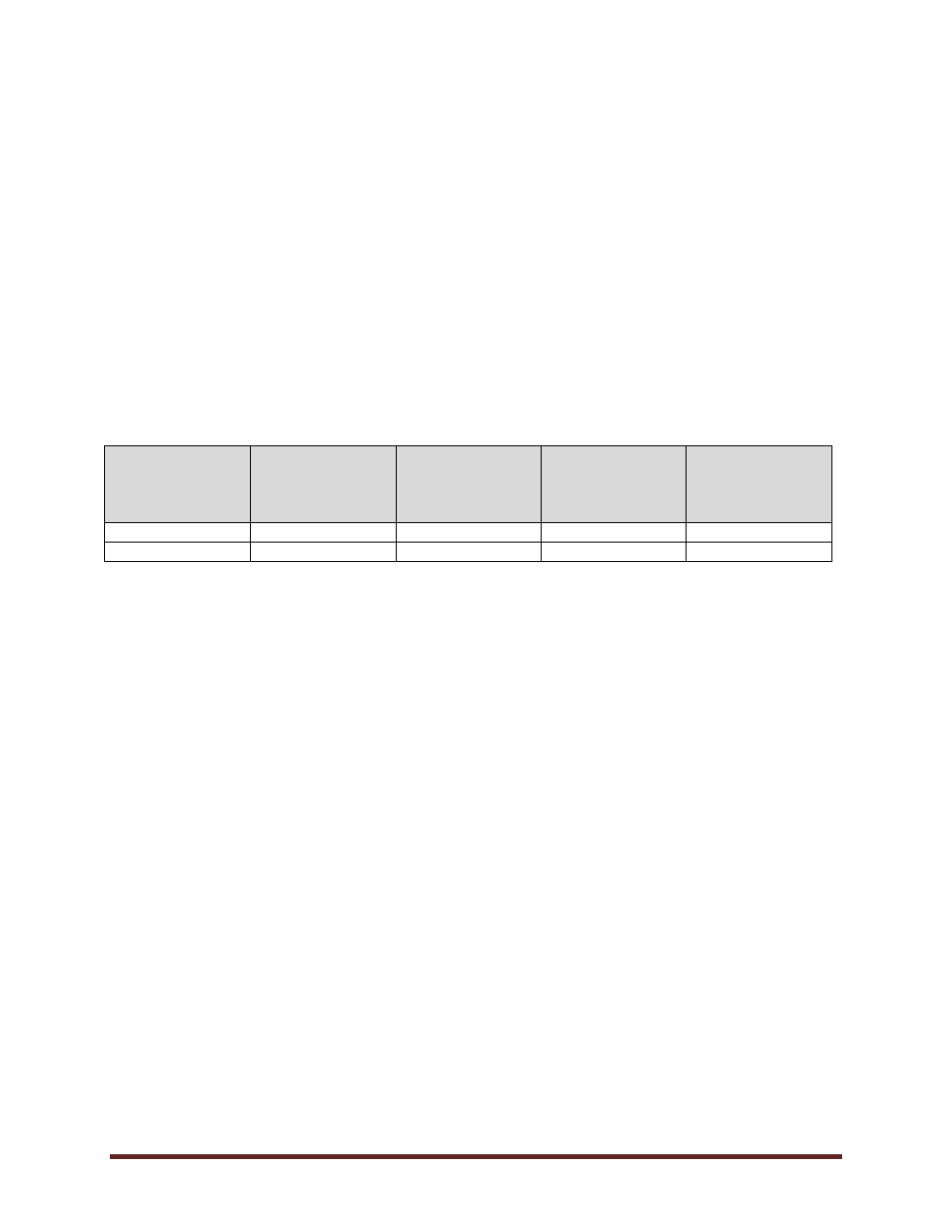
REVISION HISTORY
Date
(mm/dd/yyyy)
Description of
Change
Sections
Person who
Entered Revision
(Position Title)
Person who
Authorized
Revision
(Position Title)
04/04/2011
Policy created
05/29/2025
Policy updated
B, 2.5, 3.2, E
Biosafety Officer
Board of Governors


